Metalysis
Tuesday, February 26, 2013
Wednesday, February 20, 2013
How we get the metals in the First Place
Mining platinum and platinum group metals such as nickel, copper, and
iridium is a difficult process. Mining for these ores alone is difficult
as they only exist in small quantities around the world. Most of the
platinum exists in trace amounts around the world and is very difficult
to extract since it doesn’t exist in dense concentrations and mining for
it would result in a loss of money. A new technique is being developed
by researchers that utilize plant’s absorption of minerals in the
ground. The plants would absorb the platinum and platinum group metals
into their cells which would then be processed and the metals would be
extracted. This method of extraction is very efficient compared to
mining extraction processes. The yield is not as large but the yield
percentage is much higher. Professor James Clark, the Director of the
Green Chemistry Centre of Excellence at York, says "The trick is to
control the decomposition of the plant in a way which keeps the metal in
its nano-particulate or catalytically active form. Catalysis is being
used more and more in industrial processes and particularly for emission
control because of the demand for cleaners cars, so 'phyto-mining'
could provide a sustainable supply of catalytically active metals."
Specific species of plants such as willow, corn and mustard have adapted
over enough time to develop a resistance to being affected by these
platinum group metals and are able to absorb relatively large quantities
of these metals.
This is connected to Advanced Chemistry because the process the plants use to recover platinum and redeposit the minerals as nanoparticles in plant cells is also helping researchers and chemists to observe mechanisms involved in processes such as this. Using these mechanisms, the scientists will have better ideas on extracting metals from mine tailings that are currently uneconomical to recover. Thus, once again the mechanism of a process (not exactly just one reaction) is important.
This is connected to Advanced Chemistry because the process the plants use to recover platinum and redeposit the minerals as nanoparticles in plant cells is also helping researchers and chemists to observe mechanisms involved in processes such as this. Using these mechanisms, the scientists will have better ideas on extracting metals from mine tailings that are currently uneconomical to recover. Thus, once again the mechanism of a process (not exactly just one reaction) is important.
Monday, February 18, 2013
Coordination Complexes
In order to give a better understanding of coordination complexes, we are making this post.
First, coordination complexes are basically metal atoms surrounded by other molecules or anions, called ligands. Ligands form "dipolar" bonds with the metal, and these bonds don't belong in either molecular orbital theory, or hybridization. The bonding is similar to lewis acid and bases, where the metals are electron donors and the ligands are electron acceptors. In other words, the metals are lewis acids and the ligands are the lewis bases.
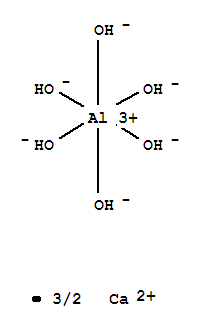
So this begs the question, how do you make the formulas for these complexes? This video gives a good idea of how this is done. A rule of thumb is that the number of ligands is twice the number of the charge of the metal. So, if you make a solution with Aluminum and hydroxide ions, the compound will be:
Anyway, here is the video:
As you can see, there is a clear connection to solutions, specifically acids and bases. There can also be a connection to electrochemistry, since electrons are being donated and accepted.
First, coordination complexes are basically metal atoms surrounded by other molecules or anions, called ligands. Ligands form "dipolar" bonds with the metal, and these bonds don't belong in either molecular orbital theory, or hybridization. The bonding is similar to lewis acid and bases, where the metals are electron donors and the ligands are electron acceptors. In other words, the metals are lewis acids and the ligands are the lewis bases.
So this begs the question, how do you make the formulas for these complexes? This video gives a good idea of how this is done. A rule of thumb is that the number of ligands is twice the number of the charge of the metal. So, if you make a solution with Aluminum and hydroxide ions, the compound will be:

Anyway, here is the video:
Tuesday, February 12, 2013
Nanocatalysts
Till now, we have mentioned coordination complexes, organometallic catalysts, and other surface catalysts. However, we have not mentioned nanomaterial based catalysts. The most obvious advantages to these catalysts is the fact that since they are so small, with order of magnitude of 9 billionths of a meter, that their surface area is very large. More surface area means more contact, so the catalysts works much more efficiently. There are many applications, and a few will be explored here.
For example, a ruthenium nano-catalysts is used for the hydrogenolysis of C-Cl bonds, which means the cleaving of the bond with the addition of hydrogen. The hydrogenolysis of halogenated (like a Cl atom) aromatic amines (benzene like rings with amine functional group) is used for the production of pesticides, herbicides and diesel fuels.
Other examples include reactions such as hydrosilyation, or as mentioned on our twitter, reactions such as the Suzuki Coupling Reaction.
Another cool fact is that research is being done on nano-particle supporters. That is, using nano particles as a base to put other nanocatalysts. Carbon nanotubes (CNTs) are examples of these supporters. The reason for this is that the high surface area of the CNTs provide for great support, and once the catalyst is on it, it can be better controlled. For example, if you want to disperse the catalyst a certain way, it can be attached to the CNTs and then dispersed in whatever way you want. This is a very interesting topic and more info can be found here.
That's it for us, hope y'all liked our post.
For example, a ruthenium nano-catalysts is used for the hydrogenolysis of C-Cl bonds, which means the cleaving of the bond with the addition of hydrogen. The hydrogenolysis of halogenated (like a Cl atom) aromatic amines (benzene like rings with amine functional group) is used for the production of pesticides, herbicides and diesel fuels.
Other examples include reactions such as hydrosilyation, or as mentioned on our twitter, reactions such as the Suzuki Coupling Reaction.
Another cool fact is that research is being done on nano-particle supporters. That is, using nano particles as a base to put other nanocatalysts. Carbon nanotubes (CNTs) are examples of these supporters. The reason for this is that the high surface area of the CNTs provide for great support, and once the catalyst is on it, it can be better controlled. For example, if you want to disperse the catalyst a certain way, it can be attached to the CNTs and then dispersed in whatever way you want. This is a very interesting topic and more info can be found here.
That's it for us, hope y'all liked our post.
Tuesday, January 15, 2013
Organocatalysts and Enzymes: Clarifications
Let's make this clear in the first line of the post: ORGANOCATALYSTS ARE NOT ENZYMES. From our previous posts. it seems their may be a confusion between the two. Yes, both have similar functions--they are catalysts. And both have an "organic flavor" to them. In fact, organocatalysts work like enzymes, but still, they themselves should not be confused with them. For example, in our last post we threw out the word chiral--without clarifying what this means. Chiral means that a structure whose mirror image is not superimposable. The following image shows this:

If you notice, when the carbon atom is attached to different groups, it is chiral, since the compound's mirror image cannot be superimposed. However, the methane is achiral, since it is connected to hydrogens in all bonds.
So we said that chirality affected function--sounds like what an enzyme does. An enzyme depends on structure as well. However, once again, enzymes are not organocatalyst. Here is a table that shows the differences between enzymes and catalysts (Note: ignore the fact that catalysts are inorganic, since organocatalysts are kind of organic). Nevertheless, enzymes have much faster reaction rates, typically very specific in their funtion, and can only work in certain conditions. Organocatalysts very rarely work in as specific conditions as enzymes. So, in conclusion, we may compare enzymes and organocatalysts. However, these are not one in the same, so sorry for any confusion.

If you notice, when the carbon atom is attached to different groups, it is chiral, since the compound's mirror image cannot be superimposed. However, the methane is achiral, since it is connected to hydrogens in all bonds.
So we said that chirality affected function--sounds like what an enzyme does. An enzyme depends on structure as well. However, once again, enzymes are not organocatalyst. Here is a table that shows the differences between enzymes and catalysts (Note: ignore the fact that catalysts are inorganic, since organocatalysts are kind of organic). Nevertheless, enzymes have much faster reaction rates, typically very specific in their funtion, and can only work in certain conditions. Organocatalysts very rarely work in as specific conditions as enzymes. So, in conclusion, we may compare enzymes and organocatalysts. However, these are not one in the same, so sorry for any confusion.
Saturday, December 29, 2012
Ligands Go Chiral!
Scientists, specifically Baihua Ye and Nicolai Cramer added biotin to a cyclopentadienyl ring,
functionalized it with rhodium, and attached it to the protein streptavidin.
What does this all do? This complex allows them to perform rhodium catalyzed
reactions to make single-enantiomer products, or products with a single
conformation. This is very important to scientists since it allows them to get products that they desire, as as we all know, a little change in structure results in a big change. Here is a link of an image of what is being described
You can see in the picture that a ring is attached to Rhodium--that is the catalyst. Furthermore, the Rh is connected to Streptavidin which is then connected to biotin. This may seem very complicated, which it is, the point is that the conformation in this picture is what makes the complex useful. It works like an enzyme, a lock and key like structure. Once again, this we see the connection of transmetal catalysts to biology and specifically enzymes. One may think that since enzymes are catalysts, that it isn't out of the ordinary for there to be such a connection between enzymes and transition metal catalysts. However, it is very hard to join these things together, as seen in our previous posts about the rhodium catalyst as well. So don't take this as a walk in the park. The emergence of organocatalysts are no minor thing in the timeline of chemistry.
Tuesday, December 11, 2012
Bang for Your Biofuel Buck?
Metal catalysts are being used to prepare new biofuels
that would improve the environment as well as producing a fuel that could be
used in place of gasoline. The catalytic metals help assist in the fermentation
technique once used to make cordite, the explosive propellant that replaced
gunpowder in bullets and artillery shells. With the addition of the metal
catalyst, researchers at the U.S. Department of Energy (DOE)'s Lawrence Berkeley
National Laboratory (Berkeley Lab) have shown that the production of acetone,
butanol and ethanol from lignocellulosic biomass could be selectively upgraded
to the high volume production of gasoline, diesel or jet
fuel.
This connects claerly to the environment, as biofuels can prove to be a great extra source of...fuel. However, are biofuels really a solution? Although the article mentions how making biofuels is great, it doesn't address the fact that biofuels still need to be burneed to be used. Its a combustion reaction...which is not good for the environment! Here it is, the combustion of ethanol and methanol (yes we are too lazy to actually type it)

As we know, the burning is exothermic so change in Enthalpy is negative (this may seem obvious but bear with me). Thus, we get energy from this reaction. However...the Carbon dioxide biproduct is not favorable. So biofuels just give us more oil, they do not solve the environmental problems. So even though the scientists use catalytic metals to produce biofuels, I would not glorify their process too much not only to show we are not biased towards all transition metal catalytic processes, but also to show that making biofuels only postpones the environmental problem.
Subscribe to:
Comments (Atom)