First, coordination complexes are basically metal atoms surrounded by other molecules or anions, called ligands. Ligands form "dipolar" bonds with the metal, and these bonds don't belong in either molecular orbital theory, or hybridization. The bonding is similar to lewis acid and bases, where the metals are electron donors and the ligands are electron acceptors. In other words, the metals are lewis acids and the ligands are the lewis bases.
So this begs the question, how do you make the formulas for these complexes? This video gives a good idea of how this is done. A rule of thumb is that the number of ligands is twice the number of the charge of the metal. So, if you make a solution with Aluminum and hydroxide ions, the compound will be:
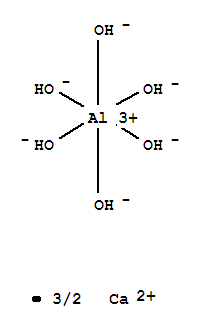
Anyway, here is the video: